Poročilo BARC Data, BI & Analytics Trend Monitor 2026 jasno sporoča vsem podatkovno usmerjenim organizacijam: razvoj umetne inteligence se pospešuje, vendar brez zaupanja vrednih, kakovostnih in upravljanih podatkov tudi najambicioznejše AI-pobude ne dosežejo ciljev. Kako te ugotovitve vplivajo na področje življenjskih znanosti, predvsem pa na farmacevtsko industrijo?
V nadaljevanju povzemamo, kaj najvišje uvrščeni trendi pomenijo za farmacevtske ekipe — in katere konkretne korake lahko organizacije sprejmejo, da se pripravijo na naslednjo fazo inteligentne, skladne avtomatizacije.
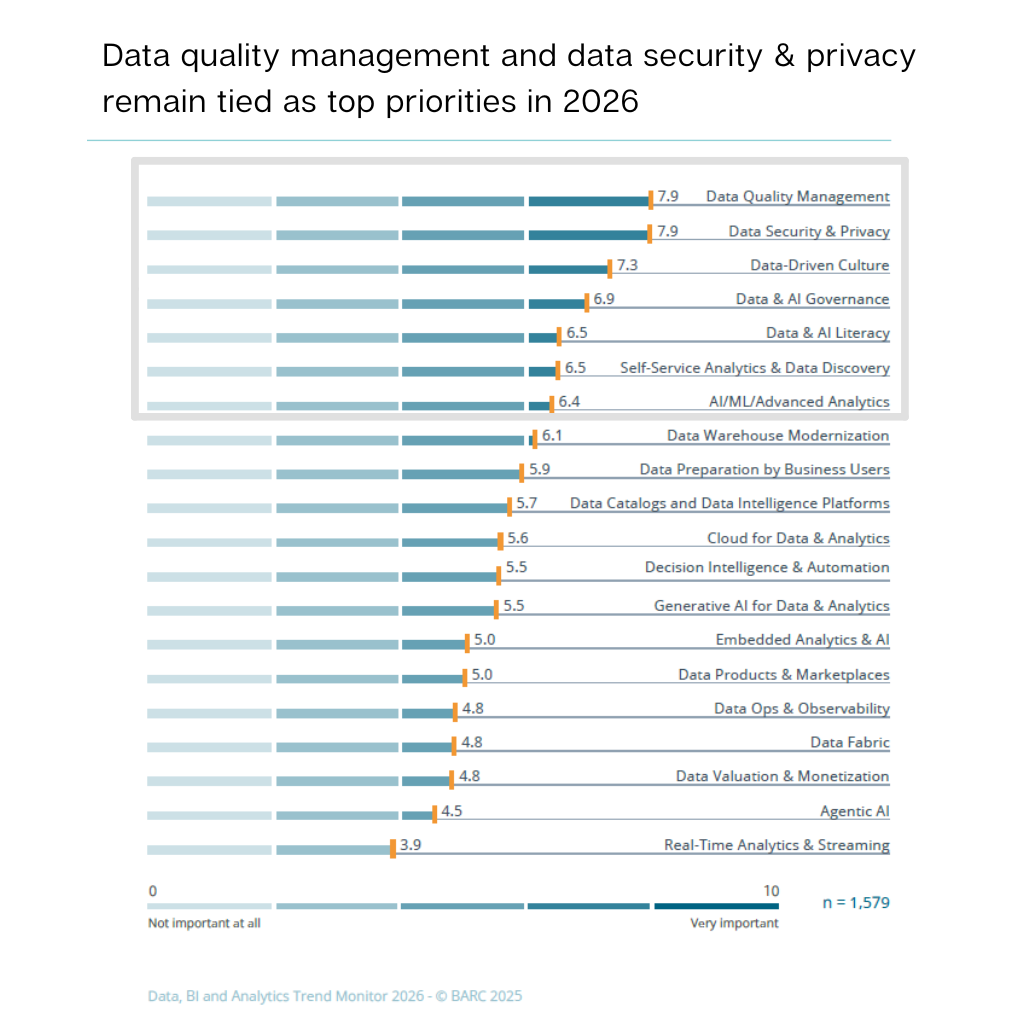
Lestvica BARC 2026 nedvoumno kaže: kakovost podatkov, varnost, upravljanje podatkov (governance) in podatkovna pismenost ostajajo najpomembnejši pospeševalci AI — še posebej v reguliranih okoljih, kot sta farmacevtska proizvodnja in razvoj. Povečana prisotnost agentne umetne inteligence, intelligence odločanja in napredne analitike še dodatno okrepi potrebo po transparentnih, revizijsko sledljivih in kontekstualiziranih podatkovnih tokovih. Zato pot do AI ni le tehnološko vprašanje, temveč predvsem izziv podatkovne pripravljenosti.